Growth is defined as an irreversible change in the size of a cell, organ or whole organism. It may also be the increase in cell number without changes in volume or weight. Commonly, growth is the increase in the amount of living material (protoplasm) which leads to an increase in cell size and ultimately cell division. The increase in protoplasm is brought about as water, carbon dioxide and inorganic salts are transformed into living material. Growth occurs only in living cells by metabolic processes involved in the synthesis of proteins, nucleic acids, lipids, and carbohydrates at the expense of metabolic energy provided by photosynthesis and respiration (7).
Differentiation is the processes involved in the establishment of localized differences in biochemical and metabolic activity and in structural organization that result in new patterns of growth (7). Differentiation of individual cells involves the system atic turning on and off of genetic control mechanisms, with mitotic processes in cell division insuring genetic continuity of all cells (7).
Whole plant development is the orderly and progressive change from seed germination through juvenility, maturity, flowering and fruiting. Environmental factors may influence developmental times or block particular stages altogether (7).
The terms growth, differentiation and development encompass the events related to the progressive unfolding of the plants genetic information in relation to environmental cues. The plant receives various internal and external stimuli that interact with the genetic information which may then change metabolic activity and influence structural organization (12).
Growth in plants is restricted to certain embryonic regions, variously called meristems, buds and cambium. These localized embryonic regions of higher plants differ from animals where growth typically occurs throughout the organism. The stems and roots have apical meristems responsible for extension growth which usually remain permanently embryonic and capable of growth for long periods. Growth in girth occurs with cell division and enlargement in cambial tissue of stems (16).
The growth and developmental patterns of plants are commonly used to classify plants into groups. Annual plants complete their entire life cycle, from seed to seed, in a single growing season, whereas biennial plants require two growing seasons. Perennials grow year after year often taking years to mature. In herbaceous perennials the roots and shoots can remain alive indefinately but the shoot system may be killed by frost. Each spring shoot growth resumes from adventitious buds at the crown of the plant. In woody perennials, both the shoots and the roots remain alive indefinitely (7).
Indeterminate plants are those whose main axes remain vegetative and in which flowers form in axillary buds. These plants have shoots which continue to grow in favorable conditions and include such vining plants as cucumbers, peas and grapes. A determinate plant's main and secondary axes terminate in a flower bud and consequently shoot elongation stops as in sweet corn, bush tomatoes, peppers, bush beans, etc. (4).
Measurement of Growth
Plant growth is often measured as a change in area, length, volume, height, wet or dry weight. These methods may not always be a satisfactory measure of growth at a particular stage of plant development, i.e., a germinating seed may show an overall loss in dry weight due to the utilization of food reserves during respiration, although the seed is definitely growing as evidenced by its emerging roots and shoots.
The relative growth rate (RGR) which is the size increase per unit interval of time has two components: the net assimilation rate (NAR) and the leaf area ratio (LAR). The NAR is the rate of increase of dry weight per unit time per unit of leaf surface whi ch is a measure of the amount of photosynthetic product going into plant material. The LAR is the ratio of leaf area to dry weight which is the measure of the proportion of the plant that is engaged in photosynthesis (12). Combined they give a relative de scription of growth over time based upon plant characteristics.
Vegetative Growth
Germination includes all the steps from the seed imbibing water until the seedling is self-sustaining. Within the seed, reserve substances are enzymatically converted into materials used in synthesis or oxidized through respiration to release energy. The seed requires water, air (oxygen), and the proper temperature range such that biochemical processes can operate (7).
A seed is considered germinated when it has produced a plant that is potentially capable of continuous growth. From the beginning of this stage, until initiation of the first flower primordium, the plant is in the vegetative stage of growth. When a plant cannot be made to flower it is said to be juvenile (7).
The juvenile growth phase is characterized by the most rapid rate of growth the plant will undergo. As well, the juvenile plant may exhibit different morphological or physiological features than a mature plant of the same species. A common feature of many juvenile plants is the ability to initiate adventitious roots readily, an ability which is often decreased or lost in mature plants. The juvenile phase varies from one to two months for annuals, to many years for woody perennials (7). The ability to infl uence the length of time a plant is in the juvenile phase is important in some circumstances. Plant propagators want to maintain juvenility in order to vegetatively propagate cuttings while flower and fruit growers want to reduce the juvenile phase. Earlier flowering and fruiting reduces production costs and allows for an earlier return on investments.
Environmental factors such as periods of long or short daylight, varying nutritional levels or supplying carbon dioxide enriched atmosphere may increase vegetative growth and if properly controlled may shorten the time to maturity. The affect that environmental and hormonal factors have on the length of juvenile phase will depend ultimately on genetic control (7).
A plant is considered mature when it becomes potentially capable of reproducing. Although a plant may be mature, flowering may not occur until environmental conditions are favorable (7).
Reproductive Growth
The vegetative stage of growth ends when the vegetative stem primordia is transformed into flower primordia. Once floral initiation has begun the process is irreversible and will continue even if environmental conditions which stimulated initiation have changed (7).
Photoperiodism is the growth response of a plant to the length of the light and dark periods. Short day plants initiate flowering only when the daylength is less than 12 hours, and include many spring and fall flowering temperate plants. Long day plants i nitiate flowering only when the daylength is greater than 12 hours, or a specific critical period. Most summer flowering plants are long day plants. Day neutral plants can initiate flowering independent of day length (7).
The flowering stimulus is formed in leaves and transported to the apical meristem in response to the photoperiod. The theory that a flowering hormone florigen is responsible for flower induction has been postulated, however, this substance has not yet been isolated. The leaves of some plants initiate flowering in response to only one cycle of the proper daylength. Most plants require many cycles of proper photoperiod in order to initiate flowering and many such as the chrysanthemum may also require the proper temperature during short days to initiate flowers (7).
Phytochrome is a blue-green pigment found in all plants. It is found in two forms; the Pr form absorbs red light (660 nm) and is converted into the Pfr form. The Pfr form absorbs far red light (730 nm) and is converted back into the Pr form. Pfr is also s lowly converted to Pr during the dark phase. The net transformation from the inactive Pr form to the active Pfr form during the course of a changing photoperiod affects the flowering mechanism (4). Interruption of the dark phase (night break) by a brief p eriod of light can inhibit flowering of short day plants and initiate flowering in long day plants. Red light (incandescent lamps) are commonly used to effectively produce night break. Phytochrome is also responsible for the initiation and inhibition of germination of some seeds, however, a light requirement is not necessary for most seeds (7).
Temperature also has a direct effect on flowering. The term vernalization is used to denote any cold temperature treatment to a plant that induces flowering (4). Many biennials require a period of low temperature to induce flowering. A plant which has been given a cold treatment can be grafted onto a nonvernalized plant, and both will flower (7). This implies that a substance is produced which passes across the graft union to induce flowering in the nonvernalized plant. The flower initiating substance has not yet been identified, however, the cold requirement of some plants has been replaced by the multiple application of gibberellic acid.
Although photoperiodism and vernalization are interrelated, the stimuli produced by each are not identical. Even after a plant has received the proper temperature for vernalization, flowering will not be initiated until the plant is exposed to the proper photoperiod (7).
Water may also affect flower initiation. Many plants show more flowering in the spring when the previous summer and fall were dry (7).
Fruit development usually occurs concomitantly with flowering. Prior to pollination the increase in fruit size is a result of cell division. The stimuli and nutrients for this growth are supplied by the plant. Pollination is the transfer of pollen from th e anther to the stigma and serves two functions; first, the inhibition of flower and fruit abscission and second, to provide the male gamete for fertilization. These two functions occur separately and even though pollination has occured and fruit set is o btained, fertilization may not take place. This may be due to the failure of the pollen to germinate or the pollen tube to grow fast enough to reach the ovary before it is shed. Pollen requires the presence of organic and inorganic substances on the pisti l to stimulate its germination. Other substances chemically attract the growth of the pollen tube and may prevent fertilization. Fruit that is set and grows without fertilization and thus does not produce viable seed is called parthenocarpic. Seedless fruit are often horticulturally desireable; however vegetative propagation may be required to continue the cultivar (7).
When fertilization occurs, the developing plant no longer depends on the parent plant for a source of growth stimuli. The stimuli now come from the developing seed. The effect of the seed on fruit development is chemically mediated. The growth regulators auxin, gibberellin and cytokinin play a role in fruit development. The concentration of these substances varies at different stages of fruit development and consequently affect fruit growth and development (7).
Food materials necessary for the developing fruit are supplied by various plant parts. The availability of nutrients and moisture will have a direct effect on fruit size. When the number of fruit set is high, the size of the individual fruits will be redu ced. Removal of some of the fruit at an early stage will allow the remaining fruit to obtain more nutrients and water to produce larger fruits. Judicious fruit thinning may lead to larger, better quality fruit and an increased profit margin.
Fruit ripening of many but not all fruits coincides with a specific physiological process, the climateric. It is characterized by a sudden rise in the respiration of the fruit resulting in a burst of carbon dioxide production. Pigment changes also occur at this time with peak ripeness occurring at the peak of the climateric or immediately thereafter (7).
Temperature has a great effect on the rate of maturation and progress of the climateric. Respiration rates increase with higher temperatures increasing carbon dioxide production and decreasing fruit life, particularly postharvest storage life (7).
Ethylene, a gaseous plant hormone, is important in fruit ripening. Ethylene is produced by the developing fruit, by rotting tissue and may be a contaminant of natural gas. When fruit is stored under conditions where ethylene is removed from the environment, ripening can be delayed (7).
Senescence refers to the processes involved in the deterioration of the plant or its organs prior to death. In annuals and biennials, senescence occurs after flowering and fruiting. In perrenials it occurs as a gradual decrease in growth and viability. Se nescence can be postponed in some plants; however, death is inevitable (7).
Genetic - The genetic compliment of a plant is acquired when the zygote is formed from male and female gametes (12). The genetic information is duplicated and passed on with subsequent cell divisions. As the plant enlarges to its mature size some genes are activated while others are inactivated. Certain genes direct the synthesis of enzymes that catalyze specific biochemical reactions required for growth and differentiation. The genes involved in protein synthesis are referred to as structural genes. Regulatory and operator genes regulate the activity of the structural genes (4). The signals that stimulate the regulatory genes are believed to be growth regulators, inorganic ions, coenzymes and environmental factors such as temperature and light.
Growth Regulators
The term hormone was developed by animal physiologists to denote naturally occurring organic substances, produced at a specific site (usually a gland), effective at low concentrations, whose action may be involved at sites far removed from their origin. The term growth regulator has been used to include all naturally occurring and synthetically produced substances that affect plant growth and development (7).
Growth hormones participate in both genetic and environmental control of growth and differentiation. The pattern of distribution of growth hormones in the plant is controlled by interactions between the environment and genetic factors in the plant (16). They may be either growth inhibitors or promotors depending on the site of action and concentration of the substance. There are 5 major types of plant hormones: auxins, cytokinins, gibberellins, abscisic acid and ethylene.
Auxins are growth hormones produced in all higher plants. They appear to be formed in the meristematic tissues of stem and root apices, young developing leaves, flowers and fruits (16). The highest rate of auxin biosynthesis is in the shoot apical region. Auxin is transported downward resulting in a concentration gradient in the various plant parts. The resultant concentration of auxin has been correlated to inhibition and stimulation of growth as well as differentiation of organs and tissues (7).
Auxins influence plant growth in many ways including cell enlargement and elongation, phototropism, geotropism, apical dominance, abscission of plant parts, flower initiation and development, root initiation, fruit set and growth, tuber and bulb formation, and seed germination. Commercially synthetic auxins are used to initiate adventitous roots from cuttings. Indolebutyric acid, indoleproprionic acid and naphthaleneacetic acid are synthectic auxins applied to the bases of stem cuttings to stimulate the initiation of adventitous roots (4).
Weed control by another synthetic auxin, 2, 4-dichlorophenoxyacetic acid (2,4-D), is widespread as a selective herbicide against broadleef weeds. Auxins are also used to increase fruit set. Use of 4-chloro-phenoxyacetic acid to increase blossom and fruit set in tomatoes is also successful. Auxins are also commonly used in tissue culture procedures to initiate rooting in explants or callus (4).
Gibberellins are a group of naturally occurring plant hormones that affect cell enlargement and division which leads to internode elongation in stems. They have a dwarf reversing response allowing certain dwarf cultivars to grow to normal height when treated with gibberellin. They affect many developmental processes, particularly those controlled by temperature and light such as seed and plant dormancy, germination, seed stalk and fruit development (7).
Gibberellins are used commercially to increase fruit size of "Thompson Seedless" grapes. They are applied at fruit set or shortly thereafter. They also promote male flower initiation in cucumbers when pollen is wanted for hybrid seed production and may overcome the cold requirement for flowering of some perennial plants (4).
Cvtokinins Drimarilv Dromote cell division but they also influence cell enlargement, tissue differentiation, dormancy, phases of flowering and fruiting and retardation of leaf senescence (4).
Cytokinins and auxins interact to affect differentiation. A high auxin to low cytokinin ratio stimulates root development, whereas a low auxin and high cytokinin ratio stimulates bud development. Equal concentrations of auxin and cytokinin results in undifferentiated tissue or callus (7).
Cytokinins are not commonly used in agriculture, however, cytokinin may be used in tissue culture to induce shoot development (4).
Ethylene is a gas that diffuses readily throughout the plant. It is produced in meristematic tissues, ripening fruits, senescing flowers and fruits and germinating seeds. The cuticular coating of the plant tends to prevent losses from the plant (4).
Synthetic ethylene-releasing compounds such as ethephon have several valuable commercial applications. Ethephon is used to ripen bananas, pineapples, melons and tomatoes, and when applied as a preharvest spray it promotes uniform ripening of apples, cherries and pineapple. It is used to increase the production of female flowers on cocumbers which develop fruits and increase yields. High concentrations of ethylene may be harmful to plants, inducing leaf abscission and hastening senescence of flowers and fruits (4).
Abscisic acid interacts with other hormones in the plant, counteracting their growth-promoting effects. It inhibits rather than stimulates plant growth. Abscisic acid promotes dormancy in seeds and is involved in leaf and fruit abscision. The abscisic acid content of leaves increases following water stress, where it induces closure of the stomata (4). Abscisic acid is expensive to synthesize and no commercial applications are as yet in use.
Greenhouse growers and nurserymen commonly use growth retardants in managing plant growth. Many synthetic compounds are available to dwarf plants, increase branching and manage flowering to produce compact flowering plants in a timely manner. Use of growth retardants is specific by species and desired result.
Plant growth and development are influenced by physical, chemical and biological components in the plants environment. Any factor in the plants' environment that is less than optimum, whether it is deficient or in excess, will limit plant growth (17).
Light
Plants respond to light of the wavelengths from 300-800 nm. Plants grown in the absence of light are said to be etiolated. Etiolated plants lack chlorophyll, are tall and spindly with long internodes and have small leaves that have failed to expand (12). Their morphological expression of etiolation is related to the effect of light on auxin distribution and synthesis (4). There are no anatomical differences in the tissues formed in the light or dark, however, light accelerates many phases of growth while inhibiting certain aspects of internode elongation (12).
Light can have an effect on the morphology of the plant. Leaves on the same plant may differ depending on whether they are sun leaves or shade leaves. Sun leaves are often thicker with extra layers of pallisade parenchyma, and shorter petioles. They are also smaller in area (12).
A plant's response to light will vary depending on the intensity, duration and wavelength of the light it receives.
Light intensity refers to the concentration of light waves striking the leaf surface (7). Light intensity has been expressed in footcandles by scientists and growers until recently. Watts per square meter or microeinstein's per square centimeter are more useful and describe energy per unit area which can be related directly to power consumption for cost analysis.
Light intensity is high where there are no clouds and little moisture in the air. Water vapor in the atmosphere absorbs radiation so light intensity is lower in cloudy or humid areas. Light intensity will vary with the elevation, latitude, season and the weather conditions affecting the amount of water vapor in the air (4).
Photoprocesses in the plant vary in the intensity of the light required to initiate the reactions and the effect of the intensity on the rate of the reaction (7). The rate of photosynthesis drops on cloudy days. However, not all plants require high light intensities. Shade plants may require as little as 1/10 full sunlight for optimum growth and higher levels may cause sun burning, scald and in severe cases death if sufficient soil moisture is not available.
Photoperiodism refers to the physiological responses of plants to variations in the duration of daylight (4). The shift from vegetative growth to reproductive growth is a response to the photoperiod. The length of the vegetative growth period can be extended by growing plants in photoperiods that do not induce flowering. Daylength may also affect the time to first flower, the number of flowers produced and the number of fruit set (12). Likewise short days and cooler temperatures initiate dormancy in many temperate zone perennial plants.
The light reactions of the plant are carried on by different pigment systems that absorb specific wavelengths of light, i.e., blue, green, yellow or red light (12). Chlorophyll absorbs that radiant energy necessary for the photoprocesses of photosynthesis (7). Chlorophyll absorbs light in the red and blue portions of the spectrum (7). Phototropism, the movement or bending of stems, leaves and flowers toward light, is triggered by blue light (4). This process is believed to occur due to the accumulation of auxin in the shaded side promoting cell growth. Thus the bending movement toward the light source is a result of increased cell growth on the shaded side (4). When leaves are subjected to high levels of radiation, they may orient themselves parallel to the energy source in order to minimize the harmful effects of the intense radiation (12).
Although incoming light in the typical greenhouse in mid-winter often does not exceed 1000-1500 footcandles in many locations, good growth of lettuce may be obtained at intensities as low as 500 footcandles. Bolting results from long days and high tempera tures so most varieties of greenhouse lettuce are not grown in late spring and early summer (18).
Early spring cucumbers, at the seedling stage, respond to supplemental light. Daylength of about 12-14 hours with 1800-2000 foot candles at the plant level should be provided. Crowding should be avoided to prevent plants from becoming spindly (18).
Tomatoes grown in the late fall or early winter should be exposed to as much light as possible during normal daylight hours. However, artificial lights should not be used to extend the daylength as tomatoes are plants which flower and fruit better if dayl ength is twelve hours or less. If artificial lights are used, at least 500 foot candles at the leaf surface should be provided. Supplementary artificial light may only be economically feasible for tomatoes at the seedling stage where a greater number of p lants can be illuminated per square foot (18).
Temperature
The temperature range that supports plant growth is generally from 40-97 degrees F (4.5-36 degrees C) (7). Optimum temperatures for growth vary with the species and the stage of development and usually fluctuates night to day.
Several growth processes are sensitive to temperature. Among these are respiration, part of the photosynthetic process, maturation, flowering, fruit ripening and dormancy (7).
Photosynthetic rates are determined mainly by light intensity, CO2 levels and temperature (11). Temperature has little effect on photosynthetic rate from 50-86 degrees F (15-30 degrees C) until light and CO2 become saturated for the photosynthetic process. At this point, an increase in temperature from 68-86 degrees F (20-30 degrees C) results in a marked increase in the photosynthetic rate (11). On warm days, midday leaf temperatures may be high and inhibit photosythetic activity (12). Not only are metabolic processes reduced at high leaf temperatures, but moisture stress, from increased transpirational losses, results in stomatal closure which decreases the supply of CO2 to the chloroplasts slowing photosynthesis.
Respiration rates increase rapidly as the temperature increases. Temperature is a controlling factor in establishing the compensation point of greenhouse crops, the point at which the rate of CO2 consumed in photosynthesis equals the rate of CO2 given off in respiration, because of its affect on respiration rate (11). As temperatures rise the level at which the compensation point occurs for a particular light level or CO2 concentration will decrease. A cessation of growth occurs when the rate of respiration increases more rapidly than the rate of photosynthesis, resulting in a depletion of food reserves (7).
Maintaining day/night temperatures at specific levels can increase yield and quality of crops. Optimum growth of many crops occurs when greenhouse temperatures are cooler at night than during the day. The response of plants to diurnal temperature fluctuations is referred to as thermoperiodicity (11).
Temperature effects on flowering may be direct or inductive (11). The effect of temperature is direct when flower initiation occurs during the period of temperature treatment. If a specific temperature induces a change within the plant which permits flowering at another time, the effect is considered to be inductive. Vernalization is the inductive effect of cold temperatures on flower initiation. Many biennials and perennials require cold treatments to induce flowering.
Root temperatures also affect the rate of plant growth. Increasing root temperatures up to about 26 degrees C (76 degrees F) may increase top growth and the uptake of inorganic ions. This is true of many hydroponically grown crops, cucumbers in particular (2).
Gases
Green plants require oxygen for normal growth and development. The energy released in cellular respiration, from the breakdown of carbohydrates and complex organic molecules, consumes oxygen and releases CO2. Most plants respire continuously, day and night, requiring a continuous supply of oxygen. Anaerobic respiration or fermentation occurs in the absence of oxygen. The products of this form of respiration are often deleterious to the plant and the energy released is relatively small compared to aerobic respiration. Roots also require oxygen for aerobic respiration which they obtain directly from the growing media. The absorption of salts and root extension are dependent upon the energy supplied from respiration. Poorly aerated growing medias result in a decrease in water absorption due to a reduction in the permeability of the root cells. After extended periods of poor root aeration the roots stop growing and are more susceptible to disease (11).
Seeds require oxygen to germinate. Seed germination is inhibited by a lack of oxygen for prolonged periods. Often thick or oily seed coats must be removed from the seed so oxygen will be available to the embryo. Compacted or water logged soils or growing media can also create an oxygen-less environment and seeds will not germinate.
Carbon Dioxide
Carbon dioxide(CO2) is a raw material required for photosynthesis. The atmospheric CO2 concentration at the plant level is the most important rate determining factor for further increases in photosynthesis and yield (18). CO2 concentrations may fall below the ambient air concentration .03% (300 ppm) in the greenhouse when weather conditions restrict ventilation or infiltration. A crop in a tightly closed greenhouse will soon deplete the CO2 concentration which reduces growth and production by slowing or stopping photosynthesis. Unless replaced, the CO2 concentration will remain at the plants compensation point, the level at which the CO2 produced from respiration equals the amount used for photosynthesis. No growth occurs at this point.
When weather conditions permit, ventilation is an effective method of maintaining CO2 concentrations at the normal air levels. However, plants respond favorably to higher CO2 concentrations, making greenhouse supplementation of CO2 an effective method of increasing plant growth (11). Although the CO2 response is dependent upon light intensity, beneficial effects are obtained over a wide range of light intensities, either natural or artificial. CO2 enrichment is of special significance in hydroponic culture as decaying organic matter in the soil, a source of CO2, is not present (18).
CO2 is commonly supplied at 800-1600 ppm via gas CO2 generators or large tanks of liquid CO2 depending upon the cost comparison between the two and the availability of the bottled carbon dioxide.
Air pollutants
Air pollution is an important problem for producers of greenhouse crops. The sources of air pollution are increasing as new industries and highways are built. This is a particular problem for horticultural operations near urban and industrial areas. Among the phytotoxic pollutants are ozone, peroxyacel nitrates, oxides of sulfur, hydrocarbons, fluorides, carbon monoxide, herbicides, fumigants, mercury vapors (do not use mercury thermometers in greenhouses), and phytotoxic gases produced from incomplete combustion of CO2 generators (7). It may be necessary for greenhouse owners to move to areas where phytotoxic gases are not present, or to grow species that are less sensitive to these substances (11).
Often leaves and flowers are first to show signs of air pollution. Unusual discolorations, spotting, twisting or turning of leaves and abortion of flowers followed by poor growth are symptoms of air pollution.
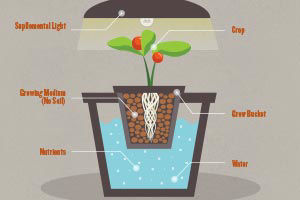
Water
Most growing plants contain about 90% water (4). Water is the medium for transfer within the plant and is the solvent system of the cell. Water is one of the raw materials for photosynthesis required for the production of new compounds. In soft tissues water pressure provides support and as plants lose water from their leaves they are cooled (7). A net loss of water will cause growth to stop and continued deficiency results in death.
A growing plant absorbs water from the soil and gives it off in transpiration. CO2 enters the plant through a film of water that surrounds the leaf and as the film evaporates it is replenished by the plant. The transpirational loss of water in exchange for CO2 is necessary for plant growth. Rapidly growing plants require large quantities of water, far in excess of that found in the plant for synthesis of new materials (7).
Moisture stress is generally detrimental to plant growth reducing both yield and quality of the crop. The degree and duration of the stress will determine how severely growth is reduced, however, growth rate may never return to the level it was before the stress (11).
The stage of growth when moisture stress occurs is also important. Moisture stress at the time of flower initiation may significantly reduce yield. Severe stress leads to premature flower, leaf and fruit drop (11).
Transpiration leads to moisture stress if moisture is not readily available to the roots. As moisture stress increases, stomates close and photosynthesis is reduced. Warm dry air has a high evaporative capacity, increasing the rate of transpiration. As we ll, the increase in leaf temperature resulting from high light intensity raises the rate of transpirational loss (11).
Poor water quality can be a major problem for growers, particulary those with hydroponic systems, due to contamination from organic and inorganic substances. Even the best domestic water supplies may contain substances that affect plant growth. Therefore, a complete water analysis is recommended for greenhouse growers. Hydroponic systems require detailed elemental analysis of irrigation waters. In order to develop an appropriate recommendation for nutrient levels in solution the concentration of existing elements in the water must be known. Adjustments can then be made in the solution for the crop to be grown. Depending on the result of the water analysis, some form of water treatment may be necessary. Water treatment may simply involve the use of a filtering system for particulate debris, or may require more sophisticated methods of ion exchange or reverse osmosis in addition to filtration. In some cases all that may be necessary is the adjustment of nutrient solution, as in hard water areas where the majority of calcium and magnesium is already provided by the water source (9).
Nutrition
Sixteen elements are considered to be essential for growth and development in higher plants. Arnon & Stout (1) considered an element essential when it 1) is required by a plant to complete its life cycle, 2) the action of the element is specific and n o other element may be substituted for it and 3) the element must exert its effect directly on growth or metabolism and not simply cause another element to be more readily available or antagonize a toxic effect of another element.
The essential elements are divided into two groups: the macronutrients, those required in relatively large quantities including carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium and sulfur and the micronutrients, those required in small quantities, including iron, chlorine, manganese, boron, zinc, copper and molybdenum (See Table 1 for Internal Concentrations of Essentials Elements in Plants).
Carbon, oxygen and hydrogen are obtained from the environment, specifically carbon dioxide or water. Along with chlorine, which is found in most water sources, these elements are generally not considered in the formulation of nutrient solutions.
Concentration in Dry Tissue
| Element | ppm | %
|
| Hydrogen | 60,000 | 6 |
| Carbon | 450,000 | 45 |
| Oxygen | 450,000 | 45 |
| Nitrogen | 15,000 | 1.5 |
| Potassium | 10,000 | 1.0 |
| Calcium | 5,000 | 0.5 |
| Magnesium | 2,000 | 0.2 |
| Phosphorus | 2,000 | 0.2 |
| Sulfur | 1,000 | 0.1 |
| Chlorine | 100 | 0.01 |
| Boron | 20 | 0.002 |
| Iron | 100 | 0.01 |
| Manganese | 50 | 0.005 |
| Zinc | 20 | 0.002 |
| Copper | 6 | 0.0006 |
| Molybdenum | 0.1 | 0.00001 |
Adapted from Salisbury F.B. and Ross C. 1969. Plant Physiology. Belmont, Calif. Wadsworth, p. 82.
Nitrogen
Nitrogen is a constituent of amino acids, proteins, coenzymes, nucleic acids and chlorophyll. Nitrogen has a great affect on plant growth and a deficiency or excess markedly affects plant growth and fruit yield (3, 8, 9, 13).
Nitrogen is a mobile element in the plant and deficiency symptoms will develop first on lower leaves as the nitrogen is removed for use in new developing leaves. The older leaves become chlorotic (turn yellow) and eventually die. Nitrogen deficiency can h ave a considerable effect on final yield if it occurs for prolonged periods during critical stages of growth (3, 8, 9, 13).
Too much nitrogen produces lush plants with dark-green thin foliage with few blossoms and fruit set is adversely affected (3, 9).
In hydroponic systems proper control of nitrogen concentration as well as the form of the element is important. Plants take up the nitrate (NO3-1) and ammonical (NH4+1) forms of nitrogen. A proper balance between the nitrate form and ammonium form is required for plant growth and also provides some degree of pH control. A ratio of 75% nitrate to 25% ammonium is satisfactory for nutrient solutions and should not exceed a ratio of 50/50 or ammonium toxicity may result (3, 9).
Most nutrient solution formulas call for 100-200 ppm nitrogen with a desired ratio of nitrate to ammonium ions at 3 or 4 to 1. Some solutions may start the nitrogen at a lower level to minimize vegetative growth and promote fruit initiation and development (9).
Nitrogen is a key essential element affecting plant growth and crop yields. Success in hydroponic growing systems may depend to a large extent on the management of this element (9).
Phosphorus
Phosphorus is a constituent of ATP, nucleic acids, phospholipids and certain coenzymes. It is very important in the plants energy transfer system and a deficiency can slow growth considerably (3, 8, 9, 13).
Phosphorus overfertilization may be a problem with soilless culture. Phosphorus toxicity may occur, interfering with the normal function of other elements such as iron, manganese and zinc (3, 8, 9).
Phosphorus deficiency reduces growth and older leaves develop a purplish color as anthoyanin pigments accumulate. Phosphorus uptake is influenced by temperature and a def iciency may be induced by cool nutrient solution temperatures (3, 8, 9, 13).
Most formulas call for 30-50 ppm of phosphorus in the form of mono- or all-hydrogen phosphate anions (HPO4- or H2PO4-1) or as phosphoric acid (H3PO4)
Potassium
Potassium acts as a coenzyme or activator of many enzyme systems. High potassium levels are required for protein synthesis and fruit production in most crops (i.e., tomato) as the demand for potassium by the developing fruit is high. A deficiency during fruiting produces fruit of significantly lower quality and size (3, 8, 9, 13).
Potassium deficiency symptoms begin as slow growth. If the deficiency becomes severe lower leaves develop a marginal chlorosis giving the appearance of burned edges (3, 8, 9, 13).
A critical balance is required between potassium, calcium and magnesium or plant stress occurs when the level of potassium is high in comparison to calcium or magnesium. High levels of potassium in solution may induce a calcium or magnesium deficiency. Care must be taken to maintain the proper balance between these three elements. Most nutrient solutions call for 200 ppm potassium in solution (9).
Calcium
Calcium is required to maintain membrane integrity and is found in cell walls as calcium pectate which cements together adjacent cell walls (13).
Calcium deficiency is generally a result of an imbalance with potassium and magnesium. It primarily affects leaf size and shape and is the cause of blossom end rot in developing fruit (3, 8, 9, 13).
A concentration of 200 ppm of calcium is common for most nutrient solution formulas. Since calcium is common in many natural water sources, a water analysis is necessary so adjustments can be made in order to avoid overfertilization which would lead to an imbalance with potassium and magnesium (3, 8, 9, 13).
Magnesium
Magnesium is an essential constituent of chlorophyll and is required for activation of many enzymes involved in the energy transfer processes. A deficiency of magnesium will seriously affect plant growth and development as photosynthesis is directly affected (3, 8, 9, 13).
Deficiency of magnesium frequently occurs due to an imbalance with potassium or ammonium ions, appearing as interveinal chlorosis developing first on older leaves. Magnesium excesses rarely occur; however, magnesium concentrations should not exceed that of calcium to maintain a proper cation balance (3, 9).
The concentration of magnesium called for in most nutrient solution formulas is 50 ppm. Irrigation waters may contain high levels of magnesium; a water analysis is necessary to manage the level properly (9).
Sulfur
Sulfur is a constituent of some amino acids and proteins, coenzyme A, thiamine and biotin (3, 9, 13).
The ratio of sulfur to nitrogen may be a better measure of the sufficiency of sulfur in the plant rather than total sulfur concentration. Deficiency symptoms appear similar to nitrogen deficiency symptoms as an overall plant yellowing or chlorosis. However, sulfur deficiency symptoms start in new leaves (because it is not translocated out of old leaves) where nitrogen deficiency symptoms, appear first in older leaves as nitrogen is easily translocated to new leaves. A plant analysis may be necessary to determine which element is deficient if the overall plant is chlorotic (3, 9, 13).
Most nutrient solution formulas call for approximately 50 ppm sulfur in the form of the sulfate anion (SO4-2). High concentration of SO4-2 ions generally do not cause any harm (9).
Boron
The role of boron in plants is not well understood although there is evidence that it is important in carbohydrate synthesis and transport. Minute quantities (<.5 ppm) are usually required by plants, and many are sensitive to higher levels of this element (3, 8, 13).
Boron deficiency will slow growth often stunting the whole plant. Fruit development will be slow and fruit quality poor (3, 9).
Boron toxicity from excess boron in the nutrient solution or boron in the water supply results in discoloration and eventual death of the leaf margins (3, 8, 9, 13).
Nutrient solution formulas usually call for about .3 ppm boron commonly in the form of borate anion (BO3-3) or boric acid (H3BO3). Well water in the western U.S. may contain toxic or near toxic levels of boron. Test irrigation waters to know (3, 9, 13).
Chlorine
Chlorine is required in photosynthesis as an enzyme activator during the production of oxygen from water (13).
Chlorine is rarely ever deficient as it is a common constituent in water and chemicals used to prepare nutrient solutions. It does not normally have to be added to the nutrition program. An excess of chlorine will result in burning of leaf margins and pre mature loss of leaves (9).
Copper
Copper acts as an electron carrier and as a constituent of certain proteins and enzymes. A copper deficiency results in plants that are stunted with chlorotic older leaves, while developing fruit are small and poorly formed (3, 8, 9, 13).
Hydroponic nutrient solutions require from 0.01 to .1 ppm copper, found in the nutrient solution as the cupric cation (Cu+2). In hydroponic growing systems, if the copper concentration gets too high root damage may result (3, 9).
Iron
Iron is required for the synthesis of chlorophyll and is an essential part of the cytochromes which serve as electron carriers in photosynthesis and respiration (3, 9, 13).
Iron deficiency appears as an intervernal chlorosis, of the younger plant tissue, which may be confused with other elemental deficiencies. A plant analysis may be necessary to determine the actual cause (9).
Iron easily complexes with many substances so the use of a chelated form of iron is generally called for when making nutrient solutions, otherwise it will combine with other elements and become an insoluble precipitate in the bottom of the solution tank. A concentration of 2-3 ppm of iron in either the ferric (Fe+3) or the ferrous (Fe+2) form must be maintained to prevent iron deficiency (3, 9, 13).
Manganese
Manganese activates some of the enzymes involved in fatty acid synthesis, DNA and RNA formation and the enzyme isocitrate dehydrogenase in the Krebs cycle. It is involved in production of oxygen from water in photosynthesis and may be involved in chlorophyll synthesis (3, 8, 9, 13).
Manganese deficiency appears as interveinal chlorosis on the younge plant tissue and may result in significant reduction in growth if severe Manganese toxicity appears similar to deficiency symptoms at first then brow spots on older plant tissue and black specks on stems and fruit develo (3, 8, 9, 13).
Nutrient solutions call for 0.5 ppm manganese in the form of manganes sulfate (3, 9, 13).
Molybdenum
Molybdenum is required for nitrogen fixation by symbiotic nitrogen fixin bacteria and for the reduction of nitrate by the enzyme nitrate reductas (3, 9).
Molybdenum deficiency may appear as nitrogen deficiency and results i restricted plant growth and flower development. Flower abortion is commo with molybdenum deficiency. A plant analysis may be necessary to determin cause of the deficiency (3, 8, 9, 13).
Nutrient solutions call for 0.05 ppm molybdenum with ammonium molybdate common source (9).
Zinc
Zinc is required for the formation of the hormone indoleacetic acid an is an enzyme activator (3, 8, 9, 13).
Zinc deficiency results in stunting of plant and leaf growth and when severe leaves die and fall off. Deficiency symptoms may be confused with th deficiencies of magnesium, iron and manganese requiring a plant analysis to determine which element is deficient (3, 8, 9, 13).
Nutrient solution formulas call for 0.05 ppm of zinc which is commonly applied in the form of zinc sulfate (9).
Nutrients are generally absorbed against concentration gradient consequently respiratory energy is required for nutrient uptake (9). In order for respiration to continue in the roots, oxygen must be available in the root zone. Roots which become totally submerged or waterlogged for long period will suffer from a lack of oxygen. This leads to slow growth, senescence ar abscission of leaves and adventitious rooting of stems (6).
The solubility of oxygen in water is low and decreases further as th solution temperature increases. Increases in root temperature (up to about 30 degrees C) increases respiration rate of the roots, further increasing oxygen demand, requiring a constant replenishment of oxygen to the nutrient solution (9).
Root zone warming for most hydroponic systems is easily manipulated to suit the crop. Warmer root temperatures increases the rate of growth (2) and the absorption and utilization of nutrients (8). Temperature also influences the growth and development of roots affecting the amount of root surface available for nutrient and water uptake (9).
The pH of the nutrient solution affects the availability of elements. The ideal nutrient solution pH is between 6.0 and 6.5. A pH below 5.0 or above 7.0 may adversely affect plant growth by altering selected nutrient availability. The micronutrients are p articularly affected with excessive uptake at low pHs and removal from the solution through precipitation at higher pHs (9).
Saline water (water containing sodium chloride) can be used in hydroponic systems if moderately salt tolerant crops such as carnations, tomatoes, cucumbers and lettuce are grown. Saline waters have an high osmotic pressure which reduces the water uptake by the roots resulting in inhibition of plant growth. A yield reduction of 10-25% can be expected, depending on the species, variety and salinity of the water (13).
Saline water also reduces the availability of certain micronutrients, especially iron, requiring additional iron to be added (13).
The total salt concentration, measured by electrical conductivity (EC) is used to monitor the status of the nutrient solution. Most plants grow well in an EC of between 1.8 and 3.5 mmhos. The EC falls as the plants absorb nutrients from the solution. However, the EC does not measure which nutrients are being depleted, and with time selected elements may accumulate as they are not removed quickly by the plants. A deficiency of these used in large quantities may be created but not determined by the EC reading which is masked by accumulated ions. Toxic levels of certain elements may develop requiring the system to be flushed and a new solution made up (5).
Nutrient solutions are being developed to overcome the problems encountered by the addition of low demand nutrient ions in the formulation of nutrient solutions. Magnesium and potassium sulfates create a build-up of low-demand nutrient ions in relatively large quantities. Use of potassium and magnesium nitrate and the addition of micronutrients in chelated forms reduce this build-up. As well, chelated micronutrients are available to plants over a wider range of solution pH. Studies performed on lettuce demonstrated this problem. The use of an EC meter to monitor nutrient solutions was not satisfactory. Although the EC remained in recommended ranges, potassium and nitrate levels were very low within two weeks, resulting in deficiency symptoms in the lettuce plants. Solutions were designed so that ions were added to the nutrient solution in ratios similar to the rate of utilization by the plant, maintaining a stable composition for longer periods of time. Growth was vastly improved (15).
More work is necessary to develop nutrient solutions adapted for growth of cucumbers, and tomatoes and selected greenhouse crops. Care should be taken to monitor the individual element content of nutrient solutions to prevent the build up of low use elements and the imbalanced depletion of highly selected nutrients. Nutrient composition should match the needs of the crop as growth proceeds at the rate imposed by the most limiting factor whether it be deficient or in excess (17).
| Nutrient | Tomatoes | Cucumbers | Lettuce |
| Normal Range | Normal Range | Normal Range | |
| Nitrogen (%) | |||
| Total N | 3.0-5.0 | 2.5-5.0 | 2.1-5.6 |
| Nitrate | 1.2-1.5 | 0.8-1.8 | 2.5-9.3 |
| Phosphorus (%) | 0.4-0.8 | 0.5-1.0 | 0.5-0.9 |
| Potassium (%) | 4.0-8.0 | 3.0-6.0 | 4.0-10.0 |
| Calcium (%) | 1.5-4.0 | 2.0-8.0 | 0.9-2.0 |
| Magnesium (%) | 0.4-1.0 | 0.4-0.8 | 0.4-0.8 |
| Sulfur (%) | |||
| Total S | 1.0-3.0 | 0.4-0.8 | 0.2-0.5 |
| Boron (ppm) | 20-60 | 40-60 | 25-65 |
| Iron (ppm) | 50-150 | 90-150 | 50-500 |
| Manganese (ppm) | 25-50 | 50-150 | 25-200 |
| Copper (ppm) | 4-6 | 4-10 | 5-18 |
| Zinc (ppm) | 15-25 | 40-50 | 30-200 |
| Molybdenum (ppm) | 1-5 | 1-3 | 0.5-3 |
Adapted from Hydroponics World: State of the Art in Soilless Crop Production, Adam J. Savage Ph.D., Editor, and Knotts' Handbook For Vegetable Growers
Table 3. Nutrient Concentrations for Tomatoes in NFT
| NO3 | 150-200 |
| NH4 | 0-200 |
| K | 300-500 |
| P | 50 |
| Ca | 150-300 |
| Mg | 50 |
| Fe | 3 |
| Mn | 1 |
| Cu | 0.1 |
| Zn | 0.1 |
| B | 0.3-0.5 |
| Mo | 0.5 |
As adapted from The Nutrient Film Technique Horticultural Review, Chris J. Graves.
| Nutrient | Concentration (ppm) |
| Nitrate | 200 |
| Phosphorus | 60 |
| Potassium | 300 |
| Calcium | 170 |
| Magnesium | 5.0 |
| Iron | 3.0 |
| Copper | 0.1 |
| Boron | 0.3 |
| Zinc | 0.1 |
| Molybdenum | 0.2 |
From Agro Dynamics Publications, Brooklyn, New York.
| pH | 5.0-6.0 |
| EC | 2.0 mmhos |
| N | 150 ppm |
| P | 35 ppm |
| Ca | 150 ppm |
| Mg | 30 ppm |
| Fe | 1.0 ppm |
| Mn | 0.75 ppm |
| B | 0.2 ppm |
| Cu | 0.2 ppm |
| Zn | 0.2 ppm |
| Mo | 0.03 ppm |
From Agro Dynamics Publications, Brooklyn, New York.
Table 6. Deficiency Symptoms for the Essential Elements in ppm
| Element | Symptoms |
| Nitrogen: | Stunted growth, foliage becomes yellow (chlorotic) starting in older leaves. Some crops (corn, tomatoes) may show a reddish color instead of yellow. |
| Phosphorus: | Plants are dwarfed with thin stems and small leaves. Anthocyanin pigments may accumulate giving plants a purplish color occurring first in older leaves. |
| Potassium: | Older leaves develop marginal browning which can extend into the leaves, and forward curling of leaves. |
| Calcium: | Shoot tips yellow and die back, young shoots have abnormal growth with eventual die-back. New leaves affected first with distorted leaf growth; roots tips die back leaving short stubby roots with black spots. |
| Magnesium: | Interveinal chlorosis developing first on the older leaves. Withering of old leaves. |
| Sulfur: | Yellowing (chlorosis) of leaves usually beginning in new leaves. Yellowing becomes off-tan in many crops. |
| Iron: | Interveinal chlorosis beginning on younger leaves. |
| Manganese: | Interveinal chlorosis on leaves near the tip of the plant. Leaves may develop necrotic lesions and drop. |
| Boron: | Die-back of shoot and root tips, stunted growth. Internal tissues may discolor or become hollow in cauliflower and beets. Leaf symptoms include curling, brittleness, wilting, chlorotic spots. |
| Zinc: | Shortened internodes, young leaves are small, may show interveinal chlorosis. |
| Molybdenum: | Interveinal chlorosis beginning on older leaves moving up to younger leaves. |
Adapted from Resh, H.M. 1983. Hydroponic Food Production, 2nd Edition. Woodbridge Press Publishing Co., Santa Barbara, Ca. pp. 335 and Bergman, Ernest L. 1985. Nutrient Solution Culture of Plants. The Pennsylvania State Univ. College of Agriculture, Extension Service Hort. Mimeo Series II:160. pp. 21.
| Nitrogen: | Foliage is heavy with many dark green leaves, few flowers and fruits. |
| Phosphorus: | Rarely occurs, no symptoms noted. May lead to a deficiency of nitrogen, potassium, zinc or copper. |
| Potassium: | Rarely occurs, expressed as magnesium deficiency. |
| Sulfur: | Reduction in leaf size, leaves may show interveinal yellowing. |
| Calcium: | Symptoms usually expressed as deficiency of potassium, magnesium, iron, manganese, or boron. |
| Magnesium: | Poor growth. |
| Iron: | Dark green foliage may develop manganese or zinc deficiency. |
| Manganese: | Chlorotic leaves with uneven chlorophyll distribution and dark brown spots. |
| Chlorine: | Burning of leaf margins becoming necrotic in time, reduced leaf size. |
| Boron | Younger leaves deformed, yellowing or necrosis of leaves beginning at leaf tip. |
| Zinc | May result in iron deficiency. |
| Copper | Reduced growth, may lead to iron deficiency. |
| Molybdenum | Rarely occurs, leaves of tomatoes turn yellow. |
Adapted from Resh, H.M. 1983. Hydroponic Food Production, 2nd Edition. Woodbridge Press Publishing Co., Santa Barbara, Ca. pp. 335. and Bergman, Ernest L. 1985. Nutrient Solution Culture of Plants. The Pennsylvania State Univ. College of Agriculture, Extension Service Hort. Mineo Series II:160. pp. 21.
Literature Cited
- Arnon, D.I. and P.R. Stout. 1939. The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physio. 14:371-375.
- Cooper, A.J. 1973. Root temperatures and plant growth. IN: Res. Rev. 4, Commonwealth Bureau of Horticulture and Plantation crops. Commonwealth Agriculture Bureau, England.
- Bergman, E.L. 1985. Nutrient Solution Culture of Plants. The Pennsylvania State University College of Agriculture, Extension Service Hort. Mimeo Series II:160. pp.21.
- Hartman, H.T., W.J. Flocker and A.M. Kofranck. 1981. Plant Science Growth, Development and Utilization of Cultivated Plants. Prentice-Hall, Inc. pp. 676.
- Ingratta, F.J., T.J. Blom and W.A. Straver. 1985. Canada:current research and developments, p. 95-102. IN: A.J. Savage (ed.). Hydroponics Worldwide State of the Art in Soilless Crop Production. International Center for Special Studies, Honolulu, Hawaii.
- Jackson, M.B. 1980. Aeration in the nutrient film technique of glasshouse crop production and the importance of oxygen, ethylene and carbon dioxide. Acta Hort., 98:61-78.
- Janick J. 1979. Horticulture Science. W.H. Freeman and Company, San Francisco. pp.608.
- Joiner, J.N. 1983. Nutrition and fertilization of ornamental greenhouse crops, pp. 380-403. J. Janick (ed.).IN: Horticultural Reviews, Vol. 5, AVI Pub. Co., Inc. Westport, CT.
- Jones, J. B. Jr. 1983. A Guide for the Hydroponic and Soilless Culture Grower. Timber Press, Beaverton Oregon. pp. 124.
- Lorenz, O.A. and D.N. Maynard. 1980. Knott's handbook for vegetable growers, 2nd Edition, A. Wiley-Interscience Pub. John Wiley & Sons, New York, NY. p. 390.
- Mastalerz, J.W. 1977. The Greenhouse Environment. John Wiley and Sons. pp. 629.
- Noggle, G. R. and G. J. Fritz. 1983. Introductory Plant Physiology, 2nd Edition. Prentice-Hall Inc. Englewood Cliffs, New Jersey. pp. 625.
- Resh, H. M. 1983. Hydroponic Food Production, 2nd Edition. Woodbridge Press Publishing Co., Santa Barbara, CA. pp 335.
- Salisbury, F.B. and C. Ross. 1969. Plant Physiology. Wadsworth, Belmont, CA. P. 422.
- Varley, J. and S. Burvage. 1981. New solution for lettuce. Grower April:19-22.
- Wareing P.F. and I.D.J. Phillips. 1970. The Control ofGrowth and Differentiation in Plants. Pergamon Press, Ltd., New York. pp.303.
- Westwood, M.N. 1978. Temperate Zone Pomology. W.H. Freeman and Co. pp. 428.
- Wittwer, S.H. and S. Honma. 1979. Greenhouse Tomatoes, Lettuce and Cucumbers. Michigan State University Press, East Lansing. pp. 225.
Courtesy of the Hydroponic Society of America. Used by permission.